Revolutionizing sepsis detection for early intervention
Loop Diagnostics is a biotech company that develops a diagnostic tool for sepsis, using an in vitro cellular immunoassay to detect it within the first hour of bloodstream infection. Their goal is to reduce disease severity and mortality rates through timely and effective treatment.
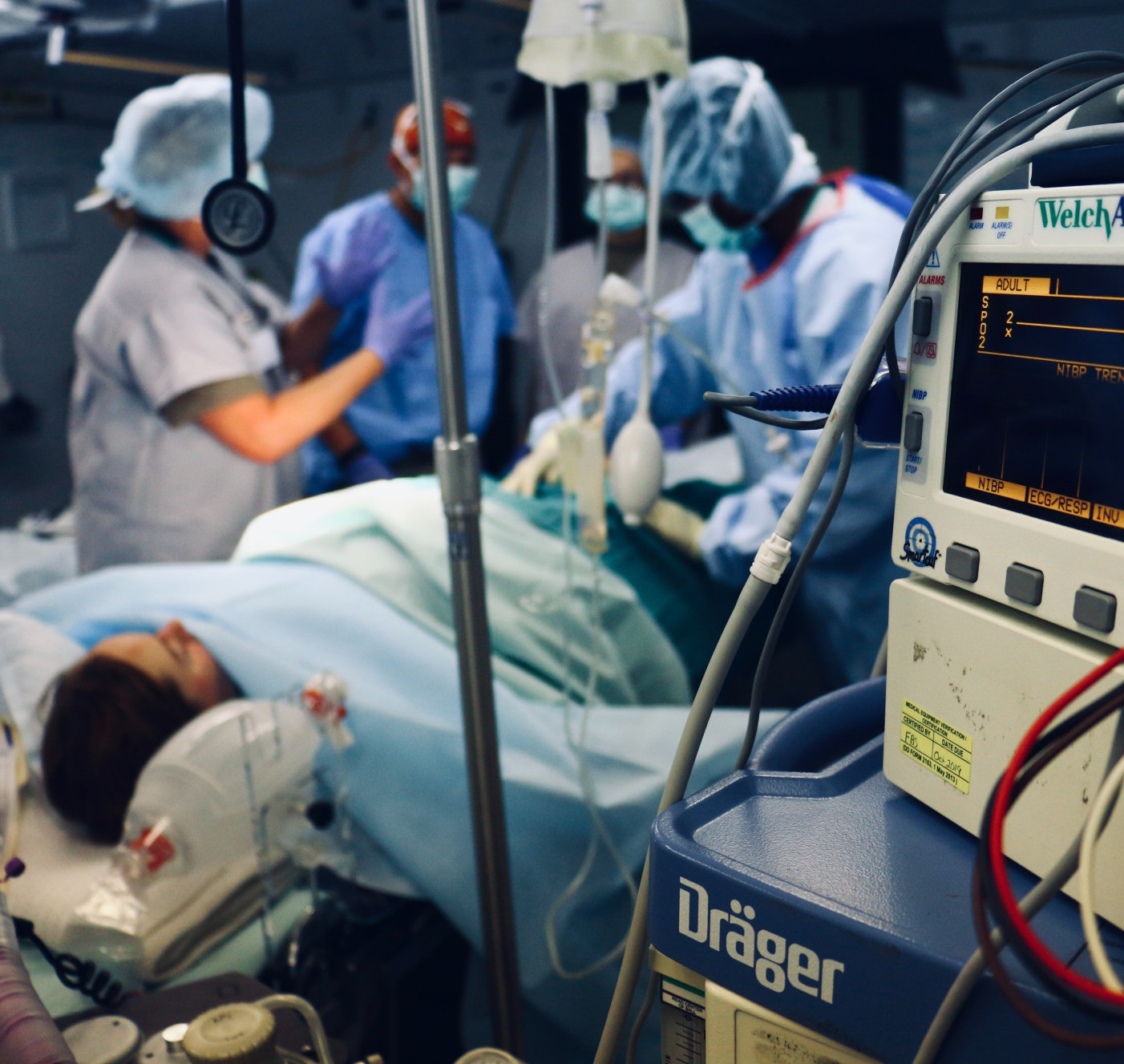
Problem
30% of mortality rate & causes 6 million deaths each year
€32 billion sepsis costs per year
Timely diagnosis and treatment are critical in managing sepsis
Complex diagnostic challenge
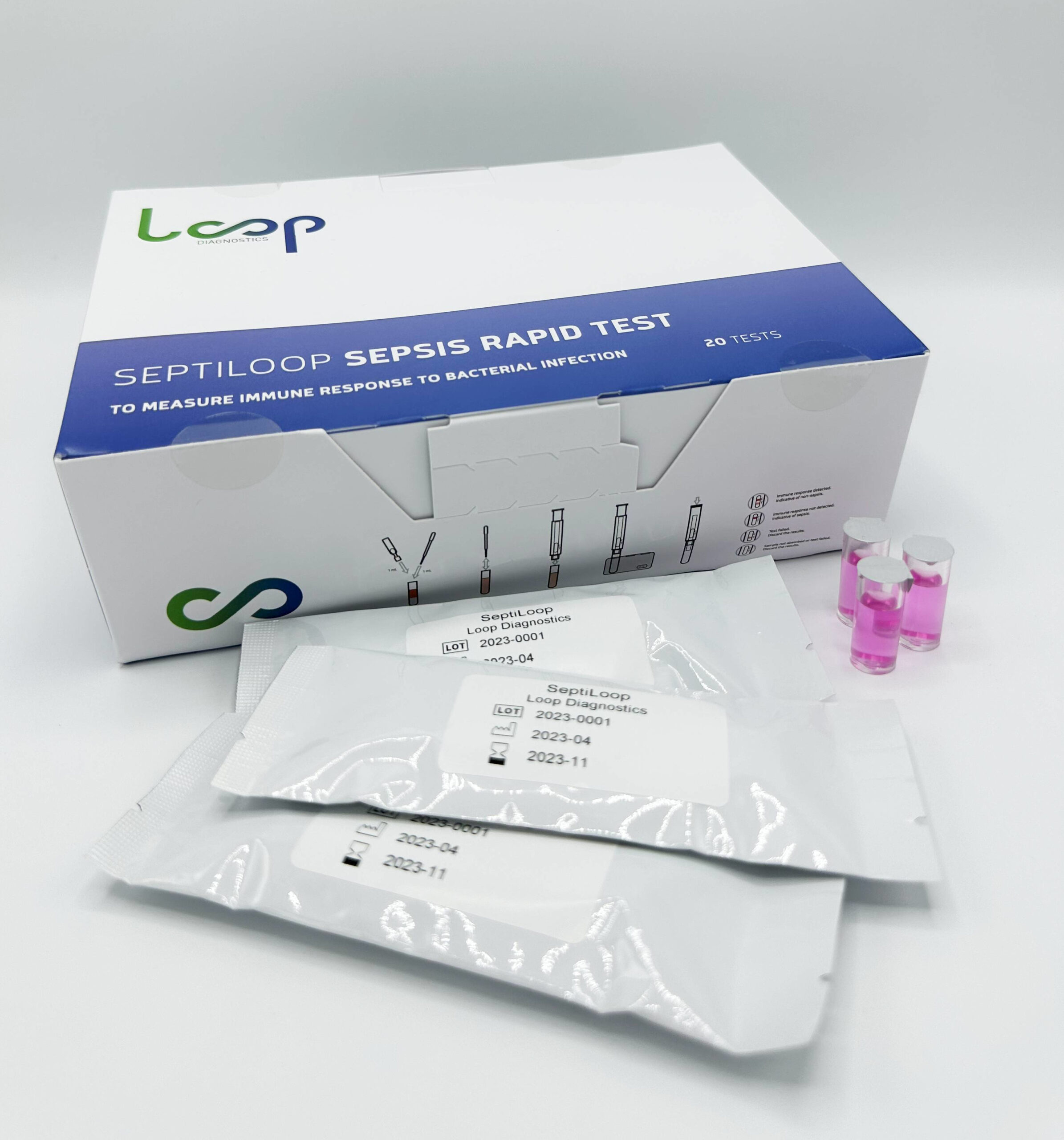
Solution
We have developed a new first-class in vitro cellular immunoassay that detects immunosuppression induced by bacteremia (including sepsis) within the first hour of bloodstream infection.
Our patented method and kit identify immune activity biomarkers with a sensitivity 3 times greater than the leading competitor. Additionally, our diagnostic process is 10 times faster than pathogen identification. Our biomarker can be detected as early as one hour after infection, significantly earlier than the current standard biomarkers. This early detection will enable healthcare providers to initiate accurate treatment at an earlier stage, reducing disease severity and minimizing the need for antibiotics or other drugs.
Line of R&D project in SMEs 04/18/SO/0017 funded by

Story
Enrique Hernández (CEO), during his Postdoctoral studies at Bellvitge Hospital in Barcelona, discovered the importance of analyzing the immune system to detect sepsis early. He recognized that traditional detection methods relied on clinical signs and symptoms, often indicating advanced disease. Hernández’s research focused on endotoxin tolerance in the innate immune system, realizing that changes in gene expression and immunological profiles could provide valuable information for early sepsis detection. Inspired by this scientific insight, he founded Loop Diagnostics, developing a technology that analyzes these factors to enable accurate and timely sepsis diagnosis. Hernández’s innovation has significantly advanced the field of sepsis detection, providing healthcare professionals with a precise tool for early intervention and improved patient outcomes.
Publications
- Assessing sepsis-induced immunosuppression to predict positive blood cultures
- Frontiers in Immunology, 4 de noviembre de 2024
- Autores: Enrique Hernández-Jiménez, Erika P. Plata-Menchaca, Damaris Berbel, Guillem López de Egea, Macarena Dastis-Arias, Laura García-Tejada, Fabrizio Sbraga, Pierre Malchair, Nadia García Muñoz, Alejandra Larrad Blasco, et al.
- PD-L1 Overexpression During Endotoxin Tolerance Impairs the Adaptive Immune Response in Septic Patients via HIF1α
- The Journal of Infectious Diseases, Julio 2017
- Autores: José Avendaño-Ortiz, Charbel Maroun-Eid, Alejandro Martín-Quirós, Enrique Hernández-Jiménez, Eduardo López-Collazo, et al.
- Human Monocytes Undergo Functional Re-programming during Sepsis Mediated by Hypoxia-Inducible Factor-1α
- Immunity, Febrero 2015
- Autores: Irina N. Shalova, Jyue Yuan Lim, Manesh Chittezhath, Enrique Hernández-Jiménez, Subhra K. Biswas, et al.
RoadMap
T1
T2
T3
T4
T5
T6
T7
T8
T9
T10
T11
T12
T13
T14
T15
T16
T17
T18
T19
T20

eduard@loopdx.com
Lab office
Cosymbio Labs - Rent shared laboratory R&D. Carrer de Roc Boronat, 31, Sant Martí, 08005 Barcelona
Phone
+34 696 17 75 93
Follow
SeptiLoop® is the only product manufactured by Loop Diagnostics that has received CE marking under the IVDR (Class C) regulation as well as UKCA certification. All other products, packaging, and product names shown on this website correspond to prototypes displayed for illustrative purposes only. These are not available for commercialization and are intended exclusively for research and performance evaluation. SeptiLoop® IFU and/or SSP can be provided upon request by emailing enrique@loopdx.com and eduard@loopdx.com.










